Male Infertility: 4 Hidden Causes and the Genetic Link
Semen Analysis and Male Fertility Parameters
Semen analysis serves as a crucial indicator of male fertility as well as male infertility. Abnormalities in its parameters can often signal underlying genetic causes (table 1). Genetic defects such as Y chromosome microdeletions, Klinefelter syndrome, or mutations affecting spermatogenesis can lead to conditions like azoospermia, oligospermia, or poor sperm motility and morphology. Understanding the genetic basis behind abnormal semen profiles not only aids in accurate diagnosis but also guides personalized fertility treatment and genetic counseling.
| Table 1: Summary Table: Semen Analysis and Male Fertility Parameters | |||
| Parameter | Normal Reference Range | Abnormal Values / Condition | Clinical Significance |
| Semen Volume | 1.5 – 6.0 mL | <1.5 mL (Hypospermia), >6.0 mL (Hyperspermia) | Indicates seminal vesicle and ejaculatory duct function |
| Sperm Concentration | ≥15 million/mL | <15 million/mL (Oligospermia), 0 (Azoospermia) | Reflects spermatogenic activity |
| Total Sperm Count | ≥39 million/ejaculate | <39 million (Subfertile), near 0 (Severe male factor) | Total reproductive potential per ejaculate |
| Progressive Motility | ≥32% | <32% (Asthenozoospermia) | Sperm’s ability to reach and penetrate the egg |
| Total Motility (Progressive + Non-progressive) | ≥40% | <40% | General motility including non-linear movement |
| Vitality (Live Sperm) | ≥58% | <58% (Necrozoospermia) | Indicates percentage of living sperm cells |
| Morphology (Strict Kruger Criteria) | ≥4% normal forms | <4% (Teratozoospermia) | Reflects structural integrity required for fertilization |
| pH Level | 7.2 – 8.0 | <7.2 (Acidic), >8.0 (Alkaline) | Reflects prostatic and seminal vesicle function |
| Leukocyte Count | <1 million/mL | >1 million/mL (Leukocytospermia) | Indicates infection or inflammation |
| Fructose Level | Positive | Negative or low (Suggests ejaculatory duct obstruction) | Energy source for sperm; reflects seminal vesicle function |
| Liquefaction Time | ≤60 minutes | >60 min (Delayed liquefaction) | Influences sperm motility and function |
| Viscosity | Low to moderate | High viscosity (Impaired motility) | Can hinder sperm movement and insemination |
| Antisperm Antibodies | Absent or <50% MAR/IBT | >50% bound sperm (Immunologic infertility) | Immune-mediated sperm dysfunction |
| Reactive Oxygen Species (ROS) | Low to moderate | High ROS (Oxidative stress) | Damages sperm DNA and membranes |
| DNA Fragmentation Index (DFI) | <15% | >30% (High DNA damage; associated with infertility) | Associated with failed fertilization, miscarriage, IVF failure |
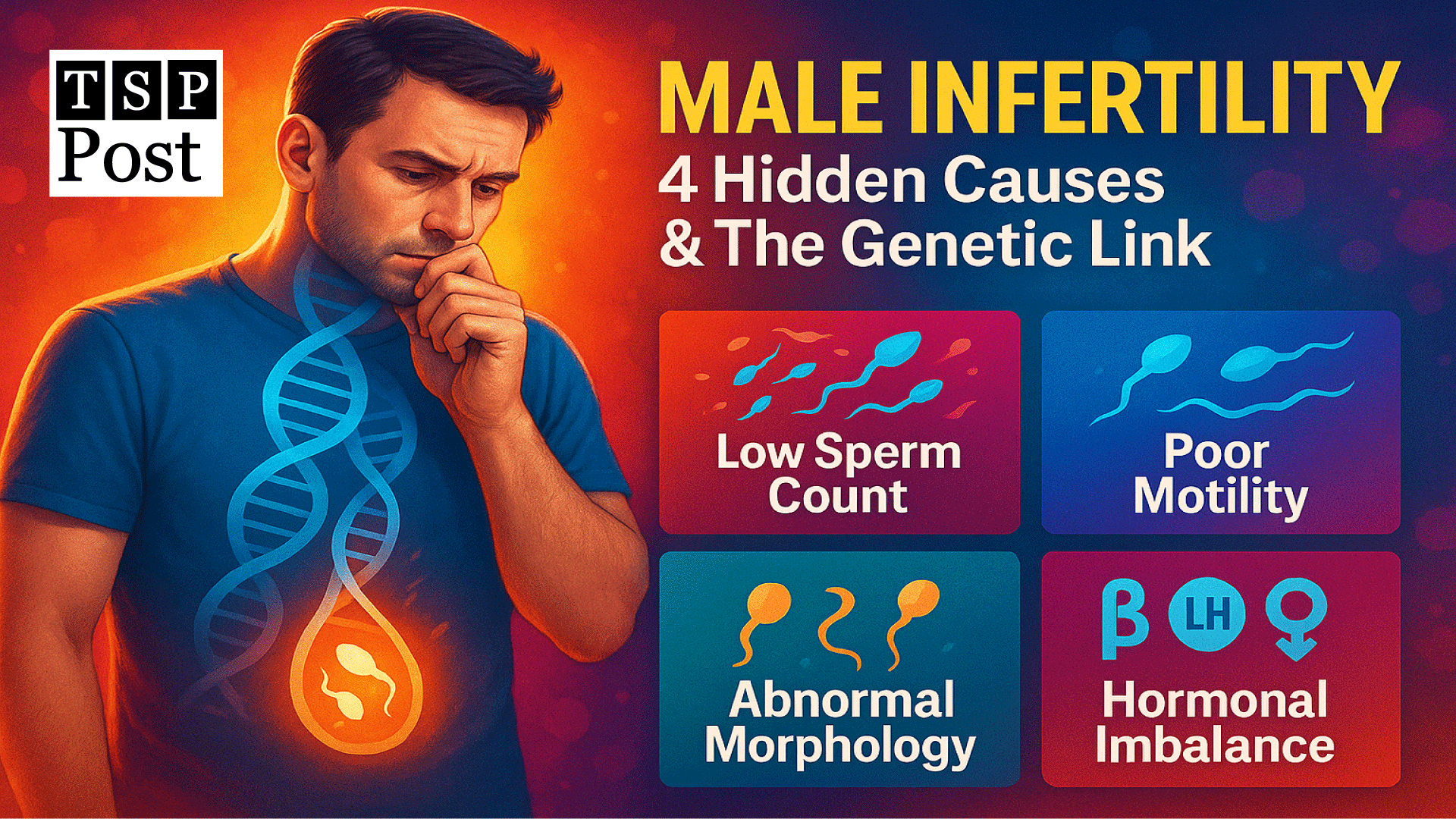
What are 4 causes of male infertility?
Infertility is a medical condition where a couple is unable to achieve pregnancy after 12 months of regular, unprotected intercourse (or 6 months if the woman is over 35). It can affect men, women, or both, and may result from genetic, hormonal, anatomical, or lifestyle factors. It affects around one in every six couples, and in nearly half of these cases, the issue lies with the male partner. While lifestyle, infections, and environmental factors are common contributors, genetic causes of male infertility are increasingly recognized as significant. In this post, we’ll explore what are 4 causes of male infertility—with a special focus on how genetic abnormalities can silently disrupt male reproductive health. Let’s discuss these causes one by one;
Low Sperm Count (Oligospermia) and Genetic Triggers
A low sperm count—or oligospermia—is one of the most common causes of male infertility. But beyond environmental and hormonal triggers, genetic mutations and chromosomal anomalies can severely reduce sperm production (table 2).
Genetic Associations:
- Y chromosome microdeletions (especially in AZF regions) can disrupt sperm production genes.
- Klinefelter syndrome (47,XXY) leads to smaller testes, low testosterone, and little to no sperm production.
- CFTR gene mutations, commonly associated with cystic fibrosis, may also block the vas deferens, reducing sperm count.
Why It Matters:
Even when lifestyle and hormonal factors are under control, an undiagnosed genetic abnormality can still prevent conception.
What Helps:
- Genetic testing (karyotyping, Y-chromosome analysis)
- Testicular sperm extraction (TESE)
- ICSI (Intracytoplasmic Sperm Injection) for assisted reproduction
Fact: About 10–15% of severe oligospermia cases are linked to genetic defects, often undetected without proper screening.
| Table 2: Low Sperm Count (Oligospermia) associated Genes/Loci | |||
| Gene / Locus | Chromosomal Location | Normal Role | Effect of Mutation |
| DAZ (AZFc) | Yq11 | RNA regulation during spermatogenesis | Deletion → Oligospermia/Azoospermia |
| USP9Y (AZFa) | Yq11 | Promotes spermatogenesis | Mutation → Sertoli-cell-only syndrome |
| RBMY (AZFb) | Yq11 | Involved in meiosis regulation | Mutation → Arrest of spermatogenesis |
| CFTR | 7q31.2 | Vas deferens development | Mutation → CBAVD, low sperm count |
| TEX11 | Xq13.1 | Meiotic progression | Mutation → Meiotic arrest, azoospermia |
| Klinefelter Syndrome | 47,XXY (karyotype) | Normal testicular function | Extra X → Small testes, low testosterone, severe oligospermia |
Poor Sperm Motility (Asthenozoospermia) – Genetics at Play
In many infertile men, the sperm count is normal—but sperm are unable to swim effectively. This is called asthenozoospermia. While lifestyle factors like heat and smoking matter, genetic mutations in motility-related proteins also play a role (Table 3).
Key Genetic Insights:
- Mutations in DNAH1, DNAH5, and CFAP43/44 genes disrupt the flagella (tail structure), impairing movement.
- Primary ciliary dyskinesia (PCD)—a genetic condition—can cause sperm flagellar defects and chronic respiratory issues.
Result:
Even if sperm reach the ejaculate, they may not be able to swim toward or penetrate the egg—limiting chances of conception.
Treatment Strategy:
- Antioxidants and lifestyle changes
- ICSI, bypassing the need for sperm motility
- Genetic counseling if PCD or similar conditions are suspected
Note: Around 20% of male infertility cases with poor motility have an underlying genetic component.
| Table 3: Poor Sperm Motility (Asthenozoospermia) | |||
| Gene / Locus | Chromosomal Location | Normal Role | Effect of Mutation |
| DNAH1 | 3p21.1 | Motor protein in sperm flagella | Mutation → Immotile sperm, poor motility |
| DNAH5 | 5p15.2 | Dynein arm assembly in cilia | Mutation → Primary Ciliary Dyskinesia |
| CFAP43 / 44 | 10q24.1 / 1p34.2 | Structure of flagella | Mutation → Flagellar disorganization, impaired motility |
Abnormal Sperm Morphology – When DNA Designs Go Wrong
Sperm morphology refers to their shape and structure. A typical sperm has an oval head, midpiece, and tail. But genetic defects during spermatogenesis can lead to headless, tailless, or multiple-headed sperm—unable to fertilize the egg (Table 4).
Genetic Link:
- Mutations in AURKC, DPY19L2, and SUN5 genes cause conditions like globozoospermia (round-headed sperm).
- Teratozoospermia (abnormal morphology) may also stem from errors in chromatin remodeling—often genetically inherited.
Fertility Implication:
Malformed sperm are less capable of swimming, penetrating the egg, or initiating fertilization.
Solutions:
- Morphology-based IVF selection techniques
- ICSI to directly inject even abnormally shaped but viable sperm
- Genetic screening before ART to prevent transmission
Did You Know? Some men have over 95% abnormally shaped sperm, yet still father children with the help of assisted techniques.
| Table 4: Abnormal Sperm Morphology (Teratozoospermia) | |||
| Gene / Locus | Chromosomal Location | Normal Role | Effect of Mutation |
| AURKC | 19q13.43 | Controls chromosomal segregation | Mutation → Globozoospermia (round-headed sperm) |
| DPY19L2 | 12q14.2 | Anchors acrosome to nucleus | Mutation → Globozoospermia |
| SUN5 | 20q11.22 | Links nucleus to flagellum | Mutation → Headless sperm (acephalic sperm) |
Hormonal Imbalance and Inherited Disorders
Hormonal balance is vital for sperm production, libido, and overall male reproductive health. While many hormonal imbalances arise from lifestyle or disease, genetic conditions often underlie serious endocrine failures (Table 5).
Genetic Causes:
- Kallmann syndrome: Inherited defect causing GnRH deficiency and low testosterone.
- Androgen insensitivity syndrome: The body doesn’t respond to testosterone despite normal or high levels.
- Congenital adrenal hyperplasia (CAH): An inherited enzyme deficiency disrupting hormone production.
Clinical Symptoms:
- Delayed puberty
- Erectile dysfunction
- Low sex drive
- Small testes or undescended testicles
Available Treatments:
- Hormonal therapy (FSH, hCG, testosterone)
- ART where hormone correction isn’t possible
- Genetic counseling for family planning
Insight: About 30–50% of men with non-obstructive azoospermia have genetic or chromosomal issues affecting hormone function and sperm production.
| Table 5: Hormonal Imbalance & Inherited Endocrine Disorders | |||
| Gene / Locus | Chromosomal Location | Normal Role | Effect of Mutation |
| KAL1 (ANOS1) | Xp22.3 | Migration of GnRH neurons | Mutation → Kallmann Syndrome, low GnRH & testosterone |
| AR (Androgen Receptor) | Xq12 | Mediates testosterone action | Mutation → Androgen Insensitivity Syndrome |
| CYP21A2 | 6p21.3 | Enzyme for adrenal steroid production | Mutation → Congenital Adrenal Hyperplasia |
| NR5A1 (SF1) | 9q33 | Testis development and steroidogenesis | Mutation → Testicular dysgenesis |
Can Genetic Male Infertility Be Treated?
Yes. Thanks to modern reproductive medicine, men with genetically linked infertility now have options:
- Testicular sperm extraction followed by ICSI
- Preimplantation genetic testing (PGT) to ensure healthy embryos
- Hormonal supplementation in genetically linked hypogonadism
Important: Genetic testing is recommended for men with:
- Azoospermia (no sperm)
- Severe oligospermia (<5 million/ml)
- Family history of infertility or syndromes
Final Words: Don’t Overlook Genetics in Male Infertility
Understanding what are 4 causes of male infertility becomes even more powerful when paired with the knowledge of genetic contributions. Whether it’s a Y chromosome deletion, CFTR mutation, or a rare inherited syndrome, genetics can influence every stage of sperm development.
Early diagnosis and genetic screening not only guide treatment but also help couples make informed reproductive choices.
FAQ – Genetics and Male Infertility
What is semen analysis and why is it important for male fertility?
Semen analysis is a key diagnostic test to evaluate male fertility. It assesses parameters like sperm count, motility, morphology, and volume. Abnormal results may indicate genetic, hormonal, or structural issues that affect a man’s ability to conceive.
Can genetics cause male infertility?
Yes, genetic abnormalities can significantly contribute to male infertility. Conditions such as Y chromosome microdeletions, Klinefelter syndrome, and CFTR gene mutations affect sperm production, motility, and structure. Genetic testing is vital in diagnosing and managing these cases effectively.
What is considered a low sperm count and what causes it?
A sperm count below 15 million/mL is termed oligospermia. Genetic mutations in genes like DAZ, USP9Y, or CFTR can cause low sperm count. Environmental, hormonal, and lifestyle factors may also play a role, but genetics often remains undetected without proper screening.
What are the effects of poor sperm motility on fertility?
Poor sperm motility, or asthenozoospermia, means sperm can’t swim efficiently to reach the egg. Genetic mutations in motility-related genes like DNAH1 and CFAP43 are common culprits. Assisted reproductive techniques like ICSI can help overcome this issue.
Can men with genetically caused infertility still have children?
Yes, many men with genetic infertility can father children through assisted reproductive technologies. Options include testicular sperm extraction (TESE), intracytoplasmic sperm injection (ICSI), and preimplantation genetic testing (PGT) to ensure healthy embryo development.