DNA Repair Genes: Maintaining Genome Stability to Prevent Cancer
Understanding the Role of DNA Repair Genes
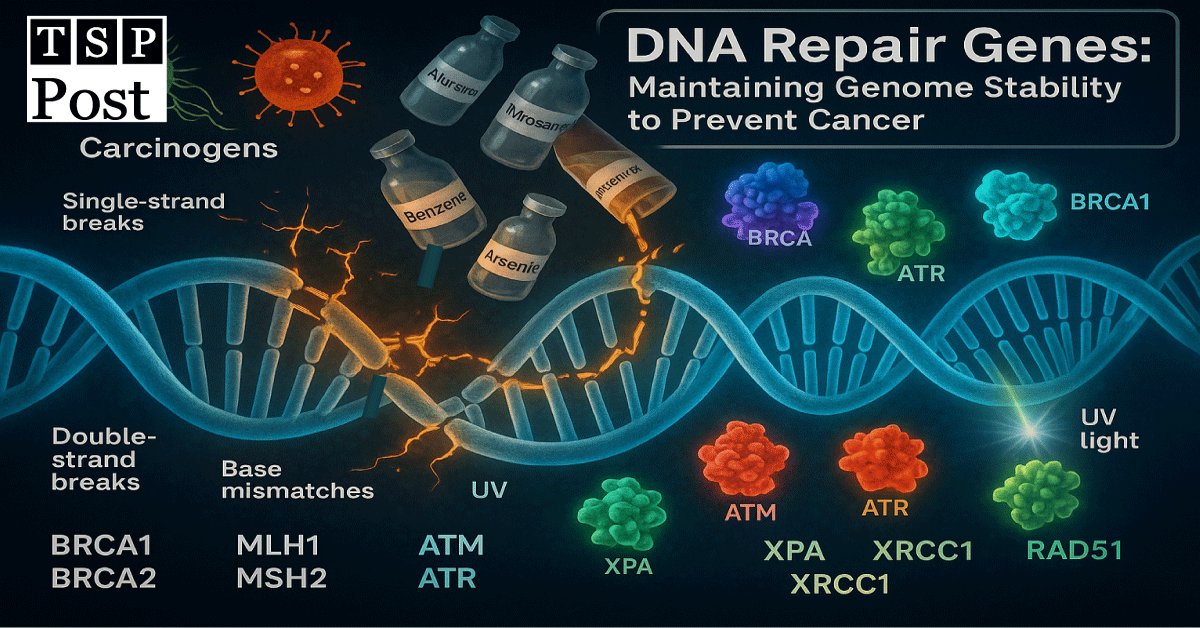
DNA replication is a high-stakes process happening billions of times across the human body daily. With so much cellular division, damage from oxidative stress, environmental mutagens, or replication errors is inevitable. As a result, DNA Repair Genes encode proteins that identify and fix this damage, acting as a defense system against mutations that can lead to cancer (Table 1). These genes ensure the integrity of the genome by:
- Continuously monitoring DNA for structural abnormalities
- Accurately correcting base-pair mismatches and double-strand breaks
- Effectively preventing permanent mutations that may lead to tumor formation
DNA Repair Genes: Functions & Disease Connections
| Gene | Full Name | Pathway | Location | Function | Abnormality / Cancer Link |
| OGG1 | 8-Oxoguanine DNA Glycosylase | BER | 3p26.2 | Removes 8-oxoguanine lesions | Linked to lung, kidney cancers |
| MUTYH | MutY DNA Glycosylase | BER | 1p34.1 | Corrects A:8-oxoG mispairs | MUTYH-associated polyposis |
| NTHL1 | Endonuclease III-like Protein 1 | BER | 16p13.3 | Removes oxidized pyrimidines | Colorectal & breast cancers |
| APEX1 | Apurinic/Apyrimidinic Endonuclease 1 | BER | 14q11.2 | Incises DNA at AP sites | Genomic instability, cancer |
| POLB | DNA Polymerase Beta | BER | 8p11.21 | Fills short gaps in repair | Mutations linked to cancer |
| LIG3 | DNA Ligase III | BER | 17q12 | Seals nicks in DNA | Overexpression in leukemia |
| XPA | Xeroderma Pigmentosum A | NER | 9q22.3 | DNA damage recognition | Xeroderma pigmentosum |
| XPC | Xeroderma Pigmentosum C | NER | 3p25 | Damage sensing | Skin cancer predisposition |
| ERCC1/ERCC4 | Excision Repair Cross-Complementation 1/4 | NER | 19q13.32 / 16p13.12 | DNA incision | Xeroderma pigmentosum, FA |
| ERCC2 | Excision Repair Cross-Complementation 2 (XPD) | NER | 19q13.32 | Helicase activity | XP, TTD, cancer risk |
| ERCC3 | Excision Repair Cross-Complementation 3 (XPB) | NER | 2q14.3 | DNA unwinding | XP, CS, TTD |
| DDB2 | Damage-Specific DNA Binding Protein 2 | NER | 11p11.2 | UV-damage recognition | XP Group E |
| CSA/CSB | Cockayne Syndrome A/B (ERCC8/6) | TC-NER | 5q12.1/10q11 | Transcription-coupled repair | Cockayne syndrome |
| MSH2/MSH6 | MutS Homologs | MMR | 2p21 / 2p16 | Mismatch recognition | Lynch syndrome |
| MLH1/PMS2 | MutL Homolog 1 / Postmeiotic Segregation Increased 2 | MMR | 3p22.2 / 7p22.1 | Mismatch repair complex | Colorectal, endometrial cancer |
| MSH3 | MutS Homolog 3 | MMR | 5q11.2 | Backup mismatch repair | Tumor susceptibility |
| PMS1 | Postmeiotic Segregation Increased 1 | MMR | 2q32.2 | Stabilizes MutL complex | Minor Lynch variant |
| BRCA1/BRCA2 | Breast Cancer 1/2 | HR | 17q21 / 13q13 | dsDNA break repair | Breast, ovarian cancers |
| RAD51 | RAD51 Recombinase | HR | 15q15.1 | Strand invasion during HR | Linked to breast cancer |
| PALB2 | Partner and Localizer of BRCA2 | HR | 16p12.2 | BRCA2 stabilization | Breast, pancreatic cancer |
| ATM | Ataxia Telangiectasia Mutated | HR | 11q22.3 | DNA damage sensing | A-T syndrome, leukemia |
| CHEK2 | Checkpoint Kinase 2 | HR | 22q12.1 | Cell cycle checkpoint | Breast, prostate cancer |
| NBN (NBS1) | Nibrin | HR | 8q21 | Part of MRN complex | Nijmegen Breakage Syndrome |
| XRCC4 | X-Ray Repair Cross Complementing 4 | NHEJ | 5q14.2 | DNA ligation | Immunodeficiency |
| LIG4 | DNA Ligase IV | NHEJ | 13q33.3 | Final ligation step | Ligase IV syndrome |
| Ku70/Ku80 | XRCC6/XRCC5 | NHEJ | 22q13.1 / 2q35 | Binds DNA ends | Radiosensitivity |
| DNA-PKcs | DNA-Dependent Protein Kinase Catalytic Subunit (PRKDC) | NHEJ | 8q11 | Activates end processing | Radiosensitive SCID |
| Artemis | DCLRE1C | NHEJ | 10p | Endonuclease for hairpins | SCID with radiosensitivity |
Mutations in these genes impair DNA surveillance and allow mutations to accumulate.
Pathways of DNA Repair: Guardians of Genomic Integrity
Base Excision Repair (BER):
Base Excision Repair is a vital molecular process that fixes tiny alterations in DNA bases, such as those caused by oxidative stress, deamination, or spontaneous hydrolysis. These subtle damages, while not distorting the DNA helix, can still trigger mutations if not promptly addressed. To counter this, specialized enzymes called DNA glycosylases initiate base excision repair (BER) by recognizing and removing faulty bases, leaving behind an abasic (empty) site. This abasic site is then cleaved by an endonuclease, followed by insertion of the correct nucleotide by DNA polymerase. Finally, DNA ligase reconnecting the strand. Functioning continuously, BER protects both dividing and resting cells from accumulating mutational errors.
For instance, the enzyme OGG1 plays a key role in eliminating 8-oxoguanine, a lesion arising from reactive oxygen species. Malfunctions in BER-related genes, such as those in MUTYH, are linked to increased risks of cancers like colorectal cancer, underlining the pathway’s importance in maintaining cellular health (Table 2).
Nucleotide Excision Repair (NER):
NER is a broad-spectrum DNA repair strategy that removes bulky distortions, including lesions from ultraviolet (UV) exposure and harmful chemicals. Damage such as thymine dimers or cross-linked bases is recognized by DNA surveillance proteins. Once identified, the affected DNA segment is cut out—typically around 24 to 32 bases—by a complex of endonucleases. DNA polymerase then synthesizes a correct patch using the opposite strand as a guide, and ligase seals the final break. Importantly, this mechanism operates through two branches: global genomic NER, which scans the entire genome for damage, and transcription-coupled NER, which prioritizes lesions that hinder transcription.
Notably, genetic defects in NER genes cause conditions like Xeroderma Pigmentosum, a disorder that dramatically increases UV sensitivity and cancer risk. Therefore, NER is essential for removing environmental DNA damage and averting mutagenesis (Table 2).
Mismatch Repair (MMR):
Mismatch Repair is the molecular proofreading system that corrects errors made during DNA synthesis (a process called Replication)—such as mispaired bases or small insertions/deletions—before they become permanent mutations. Therefore, the repair begins with recognition of mismatches by proteins like MSH2 and MLH1, followed by excision of the faulty DNA segment. DNA polymerase then accurately resynthesizes the correct sequence, and DNA ligase seals the strand. MMR safeguards replication accuracy and prevents mutational cascades. When this system fails, it can lead to genetic instability and predisposition to diseases like Lynch syndrome, which is characterized by a higher incidence of colorectal and other cancers. Additionally, many sporadic tumors exhibit microsatellite instability due to impaired MMR function (Table 2).
Homologous Recombination (HR):
Homologous Recombination is a precise and high-fidelity mechanism used to repair dangerous double-strand DNA breaks. Specifically it is predominantly active in the S and G2 phases of the cell cycle, where HR requires an intact sister chromatid to guide accurate repair. To begin with, specialized enzymes trim the broken DNA ends to generate single-stranded DNA. Then, RAD51 proteins coat the single-stranded DNA to form a nucleoprotein filament. Subsequently, this filament invades the sister chromatid, aligning with the matching sequence to enable accurate repair synthesis. Afterward, once the repair is complete, the DNA strands are resolved back into their original form.
Ultimately, HR is indispensable for preserving chromosome structure and preventing genome instability. Mutations in key HR genes such as BRCA1 and BRCA2 drastically increase susceptibility to breast, ovarian, and other cancers. Researchers actively target tumors deficient in functional homologous recombination (HR) pathways using PARP inhibitors, positioning HR as both a genomic guardian and a key therapeutic target (Table 2).
Non-Homologous End Joining (NHEJ):
NHEJ is a rapid, template-independent repair system for fixing double-strand DNA breaks, operating throughout the cell cycle but especially dominant during G0 and G1 phases. The repair begins when the Ku70/Ku80 complex binds to broken DNA ends. This complex recruits DNA-PKcs, which helps process the ends to make them ligation-ready. Subsequently, the XRCC4–Ligase IV complex joins the ends together. Though faster than HR, NHEJ is prone to small insertions or deletions, making it less accurate. Despite this, it is crucial for maintaining genome stability and for processes like V(D)J recombination in immune cells. Disruptions in NHEJ can result in hypersensitivity to radiation, compromised immunity, and higher cancer risk (Table 2).
Dysfunction in these pathways can lead to chromosomal instability and cancer progression.
Comparative Overview of Major DNA Repair Systems: From Damage to Disease
| Table 2: DNA Repair Pathways | ||||||
| DNA Repair Pathway | Damage Type | Key Mechanism | Core Proteins | Cell Cycle Phase | Function | Clinical Significance |
| Base Excision Repair (BER) | Small base lesions (oxidation, alkylation) | DNA glycosylase removes damaged base → AP endonuclease cuts backbone → DNA pol β fills gap → ligase seals | DNA glycosylases (OGG1, MUTYH), APE1, pol β, ligase III/XRCC1 | All phases | Repairs non-bulky base damage | MUTYH mutations linked to colorectal cancer |
| Nucleotide Excision Repair (NER) | Bulky helix-distorting lesions (UV damage) | Damage detected → dual incision removes ~30 bases → gap filled by pol δ/ε → ligase seals | XPC, TFIIH, XPA, XPF-ERCC1, XPG, pol δ/ε, ligase | All phases | Removes bulky DNA adducts | Defects cause Xeroderma Pigmentosum and skin cancers |
| Mismatch Repair (MMR) | Replication errors (mismatches, indels) | Mismatch recognized by MutS/MutL → excision of error strand → gap filled by pol δ → ligase seals | MSH2, MSH6, MLH1, PMS2, pol δ, ligase I | S phase | Corrects replication errors | Defects cause Lynch syndrome and MSI cancers |
| Homologous Recombination (HR) | Double-strand breaks | End resection → RAD51 filament formation → strand invasion → repair using sister chromatid | MRN complex, RAD51, BRCA1/2, PALB2, pol δ | S/G2 phases | Error-free DSB repair | BRCA mutations increase breast/ovarian cancer risk |
| Non-Homologous End Joining (NHEJ) | Double-strand breaks | Ku70/80 binds ends → DNA-PKcs processes ends → ligase IV/XRCC4 ligates | Ku70/80, DNA-PKcs, XRCC4, Ligase IV | All phases (G0/G1 predominant) | Rapid but error-prone DSB repair | Defects cause immunodeficiency and radiosensitivity |
Impact of Mutations in DNA Repair Genes
When DNA Repair Genes are mutated, the genome becomes vulnerable. Cells can no longer efficiently detect or fix errors, resulting in:
- Accelerated mutation rates
- Uncontrolled cell division
- Onset of cancer and inherited syndromes like Lynch syndrome and Fanconi anemia
These mutations can either pass from parents (germline) or develop during life (somatic), and both types sharply raise cancer risk.
Clinical Applications in Diagnosis and Treatment
DNA Repair Genes are central to modern precision oncology:
- Diagnostic Tools: Genetic testing of BRCA1/2, MLH1, and others helps assess individual cancer risk.
- Targeted Therapies: Drugs like PARP inhibitors exploit defective repair mechanisms in tumors, causing cancer cell death while sparing normal cells.
- Immunotherapy Potential: Tumors with mismatch repair deficiencies respond well to immune checkpoint inhibitors.
This personalized approach improves prognosis and reduces treatment side effects.
Conclusion: Why DNA Repair Genes Matter in the Fight Against Cancer
In the dynamic and often hostile environment within our cells, DNA Repair Genes act as sentinels—vigilantly scanning, correcting, and protecting our genetic blueprint from chaos. Their precision and functionality are essential not only for healthy development but also for cancer prevention. When these genes falter due to mutations, the results can be devastating—leading to unchecked mutation accumulation and tumor formation.
Thanks to advances in molecular biology, we now understand how these genes contribute to cancer risk, prognosis, and treatment. From BRCA-targeted therapies to genetic screenings for inherited syndromes, DNA repair pathways are now central to the future of personalized medicine.
In essence, maintaining genome stability isn’t just a cellular function—it’s a frontline defense strategy against cancer.
To explore further, visit the following link to study various aspects of cancer. Cancer Genetics
References
- Jackson, S. P., & Bartek, J. (2009). The DNA-damage response in human biology and disease. Nature, 461(7267), 1071–1078. A comprehensive review detailing DNA damage response pathways and their implications in human disease, including cancer.
- Kunkel TA, Erie DA. DNA mismatch repair. Annu Rev Biochem. 2005;74:681-710. doi: 10.1146/annurev.biochem.74.082803.133243. PMID: 15952900. An in-depth review of mismatch repair mechanisms and its role in maintaining genome integrity.
- Helleday, T., Eshtad, S., & Nik-Zainal, S. (2014). Mechanisms underlying mutational signatures in human cancers. Nature Reviews Genetics, 15(9), 585–598. Discusses various DNA repair pathways and their involvement in cancer mutagenesis.
- Wood, R. D., Mitchell, M., Sgouros, J., & Lindahl, T. (2001). Human DNA repair genes. Science, 291(5507), 1284–1289. An authoritative research article listing and characterizing human DNA repair genes.
- Hoeijmakers, J. H. (2001). Genome maintenance mechanisms for preventing cancer. Nature, 411(6835), 366–374. Classic review emphasizing the role of genome maintenance and DNA repair in cancer prevention.
For More Detailed Study
- Lindahl T, Wood RD. Quality control by DNA repair. Science. 1999 Dec 3;286(5446):1897-905. doi: 10.1126/science.286.5446.1897. PMID: 10583946.
- Sancar, A. (2016). Mechanisms of DNA repair by photolyase and excision nuclease (Nobel Lecture). Angewandte Chemie International Edition, 55(30), 8502–8527.
- Ali R, Rakha EA, Madhusudan S, Bryant HE. DNA damage repair in breast cancer and its therapeutic implications. Pathology. 2017 Feb;49(2):156-165. doi: 10.1016/j.pathol.2016.11.002. Epub 2016 Dec 26. PMID: 28034453.