Metastasis Uncovered: How Cancer Spreads at Molecular Level
Introduction
Metastasis is more than just a word in cancer biology—it’s the reason why over 90% of cancer-related deaths occur. Despite tremendous advancements in oncology, metastasis remains the most complex and poorly understood hallmark of cancer. In this blog post, we explore the molecular principles of metastasis to uncover how cancer spreads?
What is Metastasis? A Multi-Step Biological Cascade
Metastasis is the process where cancer cells break away from the original tumor, travel through the blood or lymph, and establish new tumors in distant organs. We can divide this journey into different steps to easily understand how metastasis occurs. These steps include:
- Invasion
- Intravasation
- Circulation
- Extravasation
- Colonization
Specific molecular, genetic, and epigenetic mechanisms govern each step, making metastasis not just a physical spread but a biological evolution (See Infographic Image).
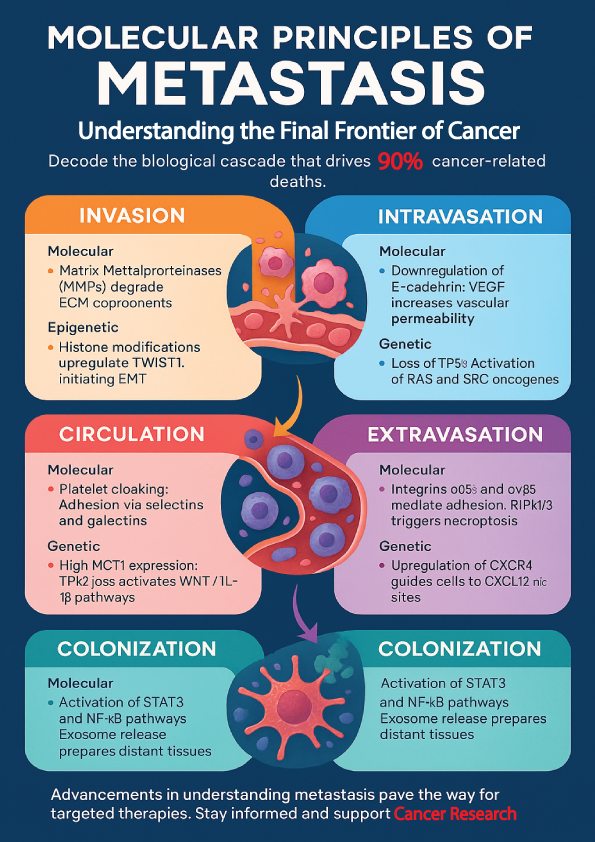
Invasion: Local Breakout of Cancer Cells
Invasion marks the initial and critical step in cancer progression, where malignant cells breach the boundaries of their tissue of origin and infiltrate surrounding normal tissues. A complex interplay of molecular, genetic, and epigenetic mechanisms orchestrates this invasive behavior. These mechanisms empower cancer cells to degrade surrounding structures and migrate into nearby tissues.
Among the key molecular players are Matrix Metalloproteinases (MMPs), which degrade components of the extracellular matrix (ECM), thereby enabling cancer cells to penetrate the basement membrane. Additionally, integrins, such as the α2β1 integrin, facilitate cell adhesion by binding to ECM components like collagen and fibronectin, assisting in cancer cell migration through tissue barriers.
On the genetic level, overexpression of genes such as MMP2, MMP9, and LOX (lysyl oxidase) significantly contributes to ECM degradation and enhanced cellular motility. These genetic alterations promote the structural remodeling necessary for invasion.
Epigenetically, histone modifications can upregulate transcription of invasion-related genes, such as TWIST1, a transcription factor crucial for initiating epithelial-to-mesenchymal transition (EMT), which enhances cell mobility and invasiveness (Table 1).
Breast cancer offers a striking example. Alterations in pyruvate metabolism actively facilitate collagen remodeling in the lungs. This remodeling supports the formation of a pre-metastatic niche and enhances the invasive capacity of cancer cells.
Invasion in Metastasis: Summary Table of Key Regulators
| Table 1 : Mechanisms of Cancer Cell Invasion | ||||
| Category | Molecular Mechanisms | Genetic Mechanisms | Epigenetic Mechanisms | Example |
| Key Players | Matrix Metalloproteinases (MMPs) degrade ECM components; Integrins (α2β1) facilitate adhesion to ECM | Overexpression of MMP2, MMP9, LOX promotes ECM degradation | Histone modifications upregulate TWIST1, initiating EMT | Breast Cancer: Altered pyruvate metabolism supports collagen remodeling in lungs for metastasis |
| Cell Behavior | ECM degradation enables cells to breach tissue boundaries and migrate | Genetic alterations enhance motility and remodeling | TWIST1 upregulation via epigenetic mechanisms increases cell motility and invasiveness | Breast Cancer: Pyruvate metabolism remodels collagen, aiding in pre-metastatic niche formation |
| Functional Impact | Facilitates tissue infiltration and migration to distant sites | Remodels ECM, enhancing cellular mobility | Promotes epithelial-to-mesenchymal transition (EMT) for invasion | Breast Cancer: Enhanced invasion and metastasis through pyruvate-driven remodeling |
Intravasation: Entry into the Bloodstream or Lymphatics
After breaching the primary tumor site, cancer cells face the critical challenge of entering the circulatory or lymphatic system—a step known as intravasation. This complex process is orchestrated by a range of molecular, genetic, and epigenetic changes that collectively enable tumor cells to penetrate the endothelial barrier and enter circulation.
Intravasation—the process is a complex event regulated by several molecular, genetic, and epigenetic mechanisms. At the molecular level, downregulation of E-cadherin, commonly triggered by epithelial-to-mesenchymal transition (EMT), disrupts cell–cell junctions and enhances cellular motility. Simultaneously, Vascular Endothelial Growth Factor (VEGF) increases vascular permeability, making it easier for tumor cells to pass through endothelial layers. Furthermore, tumor-associated macrophages (TAMs) contribute to this process by secreting factors like TNF-α and EGF, which promote endothelial disruption and tumor cell migration.
Genetically, the loss of tumor suppressor genes such as TP53 increases the likelihood of intravasation by removing key inhibitory checkpoints. In parallel, oncogenes such as RAS and SRC play a crucial role by promoting cytoskeletal reorganization and motility, further facilitating the entry of tumor cells into vessels.
On an epigenetic level, histone modifications enhance the expression of EMT-associated transcription factors such as SNAIL, ZEB1, and SLUG, which collectively drive the phenotypic changes required for successful intravasation.
A vivid illustration of this process is provided by live-cell imaging studies, which reveal that dividing tumor cells can actively disrupt endothelial barriers during mitosis, enabling their transit into the circulatory system (Table 2).
Tabular Overview of Cancer Cell Intravasation: Key Players, Behaviors, and Functional Impacts
| Table 2: Mechanisms of Cancer Cell Intravasation | ||||
| Category | Molecular Mechanisms | Genetic Mechanisms | Epigenetic Mechanisms | Example |
| Key Players | E-cadherin downregulation (via EMT) disrupts cell-cell junctions; VEGF increases vascular permeability; TAMs secrete TNF-α and EGF | TP53 loss removes inhibitory checkpoints; Oncogenes (RAS, SRC) enhance motility | Histone modifications upregulate EMT factors (SNAIL, ZEB1, SLUG) | Live-cell imaging: Tumor cells actively disrupt endothelial barriers during mitosis |
| Cell Behavior | Disruption of endothelial barriers; Increased cell motility and vessel penetration | Loss of tumor suppressor genes and activation of oncogenes increases tumor cell migration | EMT transcription factors boost motility and facilitate endothelial barrier penetration | Tumor Cells: Actively invade blood vessels or lymphatics during mitosis |
| Functional Impact | Facilitates tumor cell entry into circulation or lymphatics | Promotes cytoskeletal reorganization and enhances cellular motility | Drives the phenotypic shift for successful intravasation | Live Imaging: Disruption of endothelial barrier enables intravasation during cell division |
Circulation: Survival in the Harsh Bloodstream
Once tumor cells intravasate, they must endure the hostile environment of the circulatory system. Molecularly, circulating tumor cells (CTCs) are often cloaked by platelets, which protect them from immune surveillance and the mechanical shear stress of blood flow. Adhesion molecules such as selectins and galectins facilitate interactions with immune and endothelial cells, helping CTCs to survive and adhere. Genetically, high expression of monocarboxylate transporter 1 (MCT1) allows CTCs to metabolize lactate efficiently, supporting their survival under oxidative stress conditions. Additionally, loss of the tumor suppressor TP53 activates WNT and IL-1β signaling pathways, which enhance neutrophil recruitment and metastatic capacity. Epigenetic changes also contribute—DNA methylation patterns in CTC clusters differ significantly from those in single CTCs, influencing their ability to colonize distant organs. Breast cancer provides a striking example. Circulating tumor cell (CTC)–neutrophil clusters show heightened metastatic potential. These clusters are closely linked with rapid disease progression(Table 3).
Mechanistic Table of Cancer Cell Survival Strategies in Circulation
| Table3 : Mechanisms of Cancer Cell Survival in Circulation | ||||
| Category | Molecular Mechanisms | Genetic Mechanisms | Epigenetic Mechanisms | Example |
| Key Players | Platelets cloak CTCs to protect from immune surveillance and shear stress; Selectins and galectins aid adhesion | High MCT1 expression aids lactate metabolism; TP53 loss activates WNT/IL-1β pathways | DNA methylation in CTC clusters enhances metastatic potential | Breast Cancer: CTC–neutrophil clusters enhance metastasis and disease progression |
| Cell Behavior | Platelet coating shields CTCs; Adhesion molecules assist in survival and circulation | CTCs utilize MCT1 to metabolize lactate and survive under oxidative stress | DNA methylation patterns promote survival and organ colonization | CTC–Neutrophil Clusters: Enhanced metastatic potential and rapid disease progression |
| Functional Impact | Protects CTCs from immune detection and blood flow stresses | Enhances CTC survival and promotes metastasis | Altered DNA methylation patterns increase the ability to colonize distant organs | Live Imaging: CTCs in circulation display higher survival and metastatic efficiency |
Extravasation: Exit from the Vasculature into Distant Tissues
After surviving circulation, metastatic cancer cells must exit the bloodstream and infiltrate target tissues—a critical step known as extravasation. Molecularly, integrins such as α6β4 and αvβ5 enable tumor cells to adhere to endothelial cells and play a pivotal role in determining organ-specific metastasis. Additionally, the process of RIPK1/3-mediated necroptosis of endothelial cells creates openings in the vessel walls, allowing cancer cells to pass through. On the genetic front, upregulation of the chemokine receptor CXCR4 in cancer cells guides them toward distant organs rich in its ligand CXCL12, such as the lungs and liver. Epigenetically, the expression of extracellular matrix-modifying enzymes like matrix metalloproteinases (MMPs) is tightly regulated to support this process. A compelling example comes from studies using Necrostatin-1—an inhibitor of necroptosis—which has been shown to reduce endothelial cell death and consequently, metastatic extravasation (Table 4).
Decoding Cancer Cell Extravasation: A Comparative Mechanism Table
| Table 4: Mechanisms of Cancer Cell Extravasation | ||||
| Category | Molecular Mechanisms | Genetic Mechanisms | Epigenetic Mechanisms | Example |
| Key Players | Integrins α6β4 and αvβ5 mediate adhesion to endothelial cells; RIPK1/3 triggers necroptosis to create openings in vessels | Upregulation of chemokine receptor CXCR4 guides cells to CXCL12-rich sites (lungs, liver) | MMP expression is regulated to support vascular exit | Necrostatin-1: Inhibits necroptosis, reducing endothelial death and preventing extravasation |
| Cell Behavior | Tumor cells adhere to endothelial cells and create gaps in vessel walls for passage | CXCR4 guides cells to specific organs with abundant CXCL12 (e.g., lungs and liver) | Regulation of MMPs enhances migration and vascular exit | Live Imaging: Cancer cells use necroptosis to exit the vasculature and invade distant tissues |
| Functional Impact | Facilitates tumor cell infiltration into target tissues; Endothelial cell death allows cancer cells to breach vessels | Directs cells to distant organs for colonization | Epigenetic regulation of MMPs facilitates successful extravasation | Breast Cancer: Cancer cells utilize integrins and necroptosis to infiltrate tissues |
Colonization: Establishing a New Tumor
Colonization marks the final and most complex step of metastasis, where disseminated tumor cells establish secondary tumors in foreign tissue environments. Local cytokines like IL-6 and serum amyloid A (SAA) activate molecular pathways such as STAT3 and NF-κB. These pathways play a crucial role in supporting tumor growth. Additionally, exosomes released from primary tumors help precondition distant tissues by creating a “premetastatic niche.” Genetically, metastatic cancer cells express stemness-related genes such as SOX2 and SOX9, allowing them to resist apoptosis and sustain tumorigenesis.
In lung-tropic breast cancer cells, genes like SERPINE2 and SLPI are notably overexpressed, enhancing metastatic efficiency. Epigenetic regulation also plays a pivotal role; dormancy-associated genes such as DEC2 and NDGR1 are modulated by microenvironmental signals like BMP7, enabling cancer cells to remain latent or proliferate as needed. In brain metastasis, breast cancer cells provide a striking example of adaptation. They exploit neuronal NMDA receptors to support their proliferation and enhance resistance to chemotherapy (Table 5).
Mechanistic Breakdown of Tumor Colonization: From Molecules to Metastasis
| Table 5: Mechanisms of Cancer Cell Colonization | ||||
| Category | Molecular Mechanisms | Genetic Mechanisms | Epigenetic Mechanisms | Example |
| Key Players | Activation of STAT3 and NF-κB pathways by IL-6 and SAA; Exosome release prepares distant tissues for colonization | Expression of stemness-related genes like SOX2 and SOX9 | Regulation of dormancy genes like DEC2 and NDGR1 by BMP7 signals | Breast Cancer Metastasis to Brain: Cells exploit NMDA receptors to survive and resist chemotherapy |
| Cell Behavior | Tumor cells establish a secondary tumor in a distant tissue environment; Exosomes prepare the premetastatic niche | Overexpression of genes like SERPINE2 and SLPI enhances metastatic efficiency | Epigenetic control of dormancy or proliferation depending on microenvironment signals | Lung-Tropic Breast Cancer: Overexpression of SERPINE2 and SLPI supports efficient metastasis |
| Functional Impact | Promotes tumor growth in secondary sites; Prepares distant tissue environments for colonization | Enhances tumorigenesis by sustaining stem cell-like properties | Allows cells to either remain dormant or proliferate based on environmental cues | Brain Metastasis: Cancer cells exploit microenvironmental factors for proliferation and drug resistance |
Conclusion
Metastasis is a complex, multi-step process driven by coordinated molecular, genetic, and epigenetic changes. From invasion and intravasation to circulation, extravasation, and colonization, each step reflects cancer’s remarkable ability to adapt, survive, and thrive in new environments. Understanding these mechanisms not only unravels the biology of cancer progression but also opens the door to targeted therapies that can disrupt this deadly cascade.
Understanding Metastasis: 5 Must-Know Questions About How Cancer Spreads
What is metastasis and why does it make cancer more dangerous?
Metastasis occurs when malignant cells detach from the initial tumor and migrate to other parts of the body via the blood or lymph vessels. This aggressive spread is responsible for the majority of cancer fatalities—more than nine out of ten—since secondary tumors often complicate treatment and worsen prognosis. Because once cancer spreads, it becomes more complex, harder to detect early, and more difficult to treat. Metastatic cancer cells often acquire new properties that help them resist therapies and grow in new environments.
How do cancer cells break away and move to other parts of the body?
To metastasize, cancer cells first lose their cell-to-cell adhesion by downregulating molecules like E-cadherin, allowing them to detach from the tumor. Through a process called epithelial-mesenchymal transition (EMT), they gain mobility and invasiveness. These cancer cells release digestive enzymes, including matrix metalloproteinases (MMPs), which break down the surrounding extracellular matrix. This allows them to infiltrate adjacent tissues and enter the bloodstream or lymphatic system, facilitating their spread to distant sites in the body.
How do cancer cells survive and travel in the bloodstream?
In the bloodstream, cancer cells face immune attacks and physical stress. They survive by forming clusters with platelets and immune cells, which protect them from immune detection. These circulating tumor cells (CTCs) express survival proteins that help them resist cell death and blood shear forces, enabling them to reach distant tissues and potentially form new tumors.
What happens when cancer cells reach a new organ?
After reaching a distant organ, cancer cells go through extravasation, where they exit the blood vessels and invade the new tissue. However, not all cells can thrive there—only those that adapt to the new microenvironment can establish a secondary tumor. The new site must have favorable conditions, called the pre-metastatic niche, for these cells to survive, grow, and form metastases.
Can metastasis be treated or prevented effectively?
Although curing metastasis is challenging, early detection and targeted therapies are making progress. Treatments that block EMT, angiogenesis, or specific genetic pathways (like TP53 mutations) can help control or delay cancer spread. Advances in immunotherapy, liquid biopsies, and personalized medicine are improving how we monitor, prevent, and manage metastasis in patients.